Southlake-based HeartSciences, which closed on a $6.375M IPO in June, applies AI-based technology to an ECG to detect cardiac dysfunction—one of the earliest signs of heart disease. The company says this can’t be diagnosed by current conventional ECGs, making its MyoVista Wavelet ECG device a potential game changer.
CEO Andrew Simpson says adding this diagnostic information to an ECG “addresses a significant unmet need in the market with an estimated 100 million-plus ECG tests performed annually in the U.S.A. alone.”
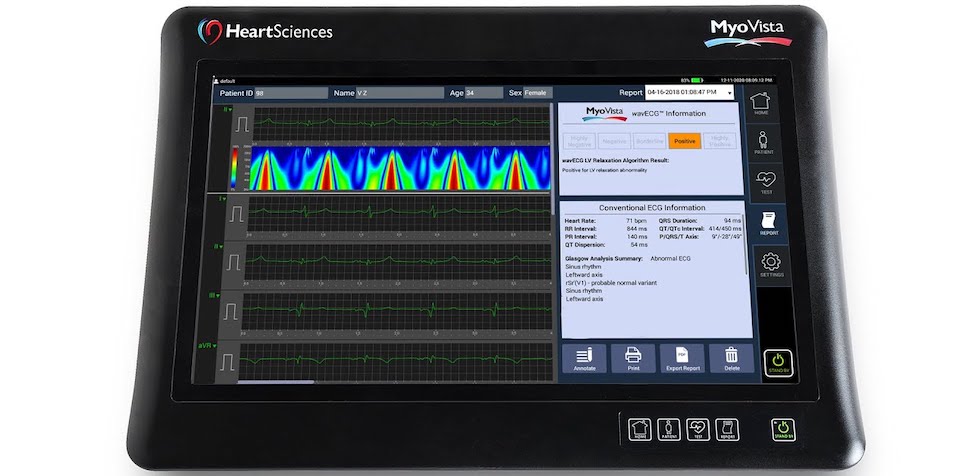
HeartSciences’ MyoVista Wavelet ECG. [Photo: HeartSciences]
In a potentially major advancement in cardiac care using ECG testing, Southlake medtech HeartSciences has been granted a patent from the U.S. Patent and Trademark Office for “ECG quantification of echocardiographic measures of diastolic function of the heart” using AI technology.
Heart Test Laboratories—which does business as HeartSciences—focuses on applying AI-based technology to an ECG (also known as an EKG) to significantly expand and improve an ECG’s clinical usefulness by detecting cardiac dysfunction, which has been recognized as one of the earliest signs of heart disease. Typically, its onset occurs when a patient is still asymptomatic, the company says..
HeartSciences’ first device, the MyoVista Wavelet ECG, leverages AI machine learning to detect cardiac dysfunction that can’t be diagnosed by current conventional ECGs. The company said that Its first algorithm is designed to provide diagnostic information related to impaired cardiac relaxation associated with diastolic dysfunction as well as all conventional ECG information—all in a single test.

Andrew Simpson, CEO of HeartSciences
Quick test can be performed in a wide range of settings
“The ECG is a ubiquitous, relatively low-cost, simple and quick test that can be performed in a wide range of clinical settings by a non-specialist clinician or clinical aide. One of the most significant needs in healthcare is the ability to detect cardiac dysfunction early,” HeartSciences CEO Andrew Simpson said in a statement. “Adding diagnostic information related to cardiac dysfunction to an ECG would not only make it a far more valuable cardiac screening tool, but also addresses a significant unmet need in the market with an estimated 100 million-plus ECG tests performed annually in the U.S.A. alone.”
Important indicator of overall heart health
Diastolic dysfunction is a problem with diastole, the first part of a person’s heartbeat. It occurs when lower heart chambers don’t relax as they should and over time, it can lead to diastolic heart failure. It’s an important indicator of overall heart health as it is impaired by all of the common pathological processes of heart disease and is a sensitive indicator of cardiovascular dysfunction. the company said.\
Currently, diastolic function of the heart must be assessed in a specialist cardiology environment most commonly via echocardiography-based imaging, HeartSciences said in a statement. ECGs have had limited, if any, role in the evaluation of cardiac dysfunction and, thus the ability to assess cardiac diastolic function using an ECG would make it a more valuable cardiac screening tool. particularly in frontline or point-of-care clinical settings, the company said.
‘A new era for ECGs’
“A growing body of published ECG research demonstrates that ECGs can have far greater clinical value and the grant of this patent reinforces our belief the HeartSciences is positioned at the forefront of what is expected to be a new era for ECGs,” Simpson said. “This latest patent is an important extension of our IP portfolio—providing significant prospective value for the company.”
Chief Operating Officer Mark Hilz said the company is intent on protecting its patent rights.
“We’re committed to patent and intellectual property protection related to our research and development efforts focused on expanding ECG clinical capabilities. We believe patent and IP protection is essential, while pursuing novel advancements for ECGs, and are proud to be awarded this valuable patent by the USPTO,” he said.
Eighth U.S. patent to date; IPO closed in June
This is the company’s eighth U.S. patent to date, and it brings the company’s total granted patents, including international, to 18. HeartSciences said it also has received allowances of further applications by the European Patent Office and Israel with several additional pending patent applications in multiple international jurisdictions including Brazil, Canada, India, South Korea, Mexico, and the United Arab Emirates.
In June, the company closed on a public offering of $4.25 per share for aggregate gross proceeds of roughly $6.375 million.
At the time, HeartSciences said it expected to use the net proceeds from the offering primarily to fund FDA clearance for the MyoVista device, including completion of the pivotal clinical validation study, and for working capital and general corporate purposes.
Founded in 2008
HeartSciences was founded in 2008 with the mission of providing accurate, affordable screening tools for the early detection of heart disease through the continuous development of innovative electrocardiography.
Known as the “silent killer,” heart disease is a leading cause of death globally. Most patients affected by heart disease are unaware and asymptomatic. Identifying these asymptomatic patients continues to be a major challenge in healthcare.
According to the Centers for Disease Control, roughly 697,000 people in the U.S. died from heart disease in 2020, accounting for 1 in every 5 deaths. Heart disease costs the United States about $229 billion each year from 2017 to 2018, the CDC said, including the cost of healthcare services, medicines, and lost productivity due to death.